Table of Contents
Unit 1: Atomic Structure and PropertiesUnit 2: Molecular and Ionic Compounds
Unit 3: Intermolecular Forces and Properties
Unit 4: Types of Chemical Reactions
Unit 5: Reaction Rates
Unit 6: Energetics
Unit 7: Introduction to Equilibrium
Unit 8: Introduction to Acid and Base
Unit 8: Gibbs Energy and Cell
Mastering Reaction Rates
Introduction to Reaction Rates
Reaction rates quantify the speed at which reactants are transformed into products in a chemical reaction. The rate of a reaction is typically expressed as the change in concentration of a reactant or product per unit time. It can be either positive or negative, depending on whether the concentration is increasing or decreasing.
Several factors can influence the rate of a chemical reaction, including:
Concentration of Reactants
As the concentration of reactants increases, the frequency of collisions between particles also increases, leading to a higher reaction rate.
Temperature
An increase in temperature typically increases reaction rates, as it provides more energy to the reacting molecules, leading to more frequent and energetic collisions.
Surface Area
Increasing the surface area of a solid reactant exposes more of its particles to the reaction, leading to a higher reaction rate.
Pressure
For gaseous reactions, an increase in pressure generally leads to an increase in the reaction rate, as it effectively increases the concentration of reactants.
Presence of Catalysts
Catalysts can speed up reaction rates by lowering the activation energy required for the reaction to occur.
Collision Theory
The collision model, also known as collision theory, is a fundamental concept in AP Chemistry that describes the process by which chemical reactions occur. This theory posits that chemical reactions take place when reactant molecules collide with one another with sufficient energy and proper orientation. In this article, we will delve into the details of the collision model, including the factors that influence reaction rates, activation energy, and the role of catalysts in chemical reactions.
Rate Laws and Reaction Order
Rate laws express the relationship between the reaction rate and the concentrations of reactants. A general rate law can be written as:
Rate = k[A]<sup>m</sup>[B]<sup>n</sup>
Where:
- Rate represents the reaction rate
- k is the rate constant
- [A] and [B] are the concentrations of reactants A and B
- m and n are the reaction orders with respect to A and B
The overall reaction order is the sum of the individual orders (m + n).
Determining Rate Laws Experimentally
To determine the rate law for a reaction, experiments are conducted to measure the rate of the reaction under various conditions. By comparing the rate data, the reaction orders can be determined.
Method of Initial Rates
In this method, the initial rate of the reaction is measured for different initial concentrations of reactants. By comparing these initial rates, the reaction orders can be deduced.
Isolation Method
This method involves isolating one reactant by using a large excess of the other reactants. By doing so, the reaction order for the isolated reactant can be determined.
Integrated Rate Laws and Half-Life
Integrated rate laws provide a mathematical relationship between the concentration of a reactant and time. Depending on the reaction order, the integrated rate law will have a different form:

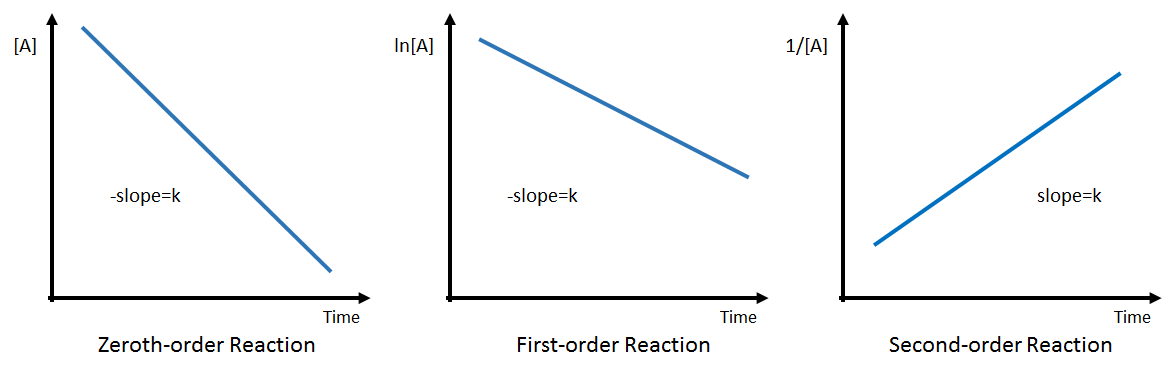
Zero-Order Reactions
For zero-order reactions, the integrated rate law is:
[A]t = [A]0 – kt
Where:
- [A]t is the concentration of reactant A at time t
- [A]0 is the initial concentration of reactant A
- k is the rate constant
- t is the time elapsed
First-Order Reactions
For first-order reactions, the integrated rate law is:
ln([A]t) = ln([A]0) – kt
Second-Order Reactions
For second-order reactions, the integrated rate law is:
1/[A]t = 1/[A]0 + kt
Half-Life
The half-life (t1/2) of a reaction is the time it takes for the concentration of a reactant to decrease to half its initial value. Depending on the reaction order, the half-life has a different expression:
- First-order: t1/2 = 0.693 / k
Reaction Mechanisms and Rate-Determining Steps
Reaction mechanisms describe the series of elementary steps that make up a chemical reaction. Each step has its own rate law, and the overall reaction rate depends on the slowest step, known as the rate-determining step.
Catalysts and Reaction Rates
Catalysts are substances that increase the rate of a reaction without being consumed in the process. They work by providing an alternative reaction pathway with a lower activation energy, allowing the reaction to proceed more quickly. Catalysts can be classified as either homogeneous or heterogeneous, depending on whether they exist in the same phase as the reactants.
Stepwise Reaction
A common example of a stepwise reaction is the reaction between hydrogen and iodine to form hydrogen iodide:
H2 + I2 → 2HI
The reaction mechanism for this reaction involves two elementary steps:
Step 1: H2 → 2H (rate constant k1)
Step 2: H + I2 → 2HI (rate constant k2)
In this example, the first step is the homolytic cleavage of the H2 molecule to form two hydrogen atoms. The second step involves the reaction between a hydrogen atom and an I2 molecule to produce two molecules of hydrogen iodide. The overall reaction is the sum of the two elementary steps.
It is important to note that the rate-determining step (RDS) is the slowest step in a stepwise reaction. The rate of the overall reaction is dictated by the rate of the RDS. In this example, if step 2 is the slower step, the rate of the overall reaction would depend on the rate constant k2 (Rate expression , R = K[H] )and the concentrations of H and I2